Stephanie L. Gupton, Ph.D.
Professor

Dept of Cell Biology & Physiology
UNC Neuroscience Center
UNC Lineberger Comprehensive Cancer Center
- B.S. North Carolina State University, 2001
- Ph.D. Scripps Research Institute, 2006
- Post-Doc Massachusetts Institute for Technology, 2006-2011
- Joined the department in 2011
111 Mason Farm Road
Campus Box 7090
Lab: 4332 MBRB
Office:4340B MBRB
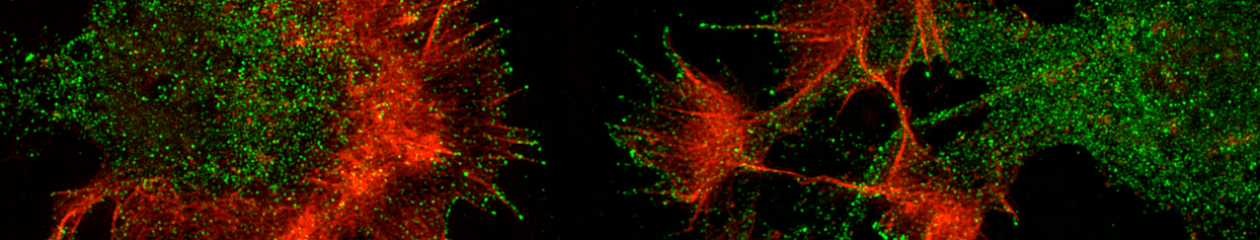
Developing neurons acquire a complex, polarized morphology, which underlies their specialized physiology and is essential to the formation and functional connectivity of the nervous system. This entails concerted insertion of membrane material to fuel plasma membrane expansion and localized cytoskeletal reorganization to directionally extend the plasma membrane. Our goal is to define mechanisms by which distinct neuronal guidance cues influence the membrane trafficking and cytoskeletal machineries that steer morphogenesis and consequently, connectivity and behavior. We do this in a supportive, engaging, and fun environment of scientific discovery and training.
Vesicle fusion is regarded as the primary source of membrane material; we define mechanisms that influence the organization and mode of exocytic delivery. Filopodial protrusions are regarded as sensors for guidance cues that initiate axon turning and branching responses; we examine the local regulation of actin dynamics required to generate and support filopodial protrusions. We use dissociated murine embryonic cortical neurons as a model, as they respond to guidance cues and recapitulate neuronal morphogenesis in vitro. The specialized protein repertoire of developing neurons is responsible for neuronal morphogenesis and response to guidance cues. We exploit unbiased proteomics with mass spectrometry in cultured neurons to identify interaction partners and post-translational modifications of neuronal proteins. We utilize a range of state-of-the-art light microscopy techniques along with computational image analysis, computational modeling, and molecular genetics, to define the mechanisms controlling the dynamics and architecture of the actin cytoskeleton within filopodia and the mode and organization of vesicle fusion with the plasma membrane. This is coupled with microfluidicis to produce stable gradients of guidance cues to rigorously investigate axon guidance mechanisms. We employ neuroanatomical and behavioral approaches to reveal the in vivo consequences of the mechanisms we define in vitro.